Excellence in Biologics Analysis

The Octet BLI system provides real-time, label-free analysis of affinity, kinetics, and antibody/protein concentration. Experiments are done with Dip and Read™ sensors and standard micro-well plates instead of chip-trays as in typical SPR kinetics. BLI is thus particularly suited for characterization of biologics/antibodies in crude mixtures, because no fluidics are involved.
All parameters that can be measured with Octet BLI are listed below:
Affinity
LIGAND BINDING
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
ΔH
Binding enthalpy. KD values at different temperatures can be used to obtain the binding enthalpy of an interaction via vant-Hoff-plots.
Kinetics
BINDING KINETICS
ka (kon)
Association rate constant. Provides information on how fast complexes form; can be used for KD determination.
kd (koff)
Dissociation rate constant. Provides information on how fast complexes dissociate; can be used for KD determination.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
Biologics
SAMPLE QUANTITATION
c
Concentration of biological macromolecules (e.g. proteins, antibodies, antibody fragments) in supernatants, production mixtures, or crude lysates
BLI provides real-time, label-free affinity and kinetics for biologics
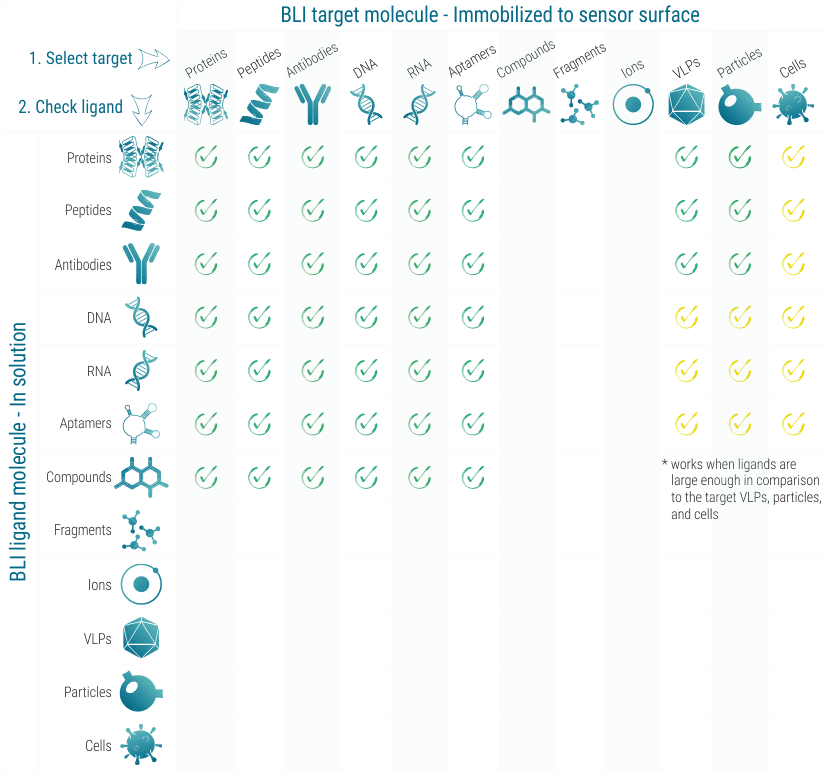
BLI works with many classes of target molecules, as long as they can be coupled to a sensor surface. For this, various coupling strategies and chemistries are available. The ligand molecule stays in solution. As long as the ligand molecule is large enough (>150 Da), even small ligands like compounds can be analyzed for their binding kinetics.
Binding kinetics
Steady-State Affinity
Sample quantification
BLI can help quantifying target concentrations in complex biological liquids such as serum or cell lysate.
Epitope sites
BLI allows for discriminating between antibodies that bind the same antigen but not the same epitope on the antigen.
- Antibody-antigen characterization
- Protein-protein interactions
- Antibody off-rate ranking
- Drug discovery (compound based)
- Antibody-antigen interactions
- Antibody affinity ranking
- Protein-protein interactions
- Drug discovery (compound based)
- Antibody quantification in supernatants
- Clone selection
- Epitope binning
Advantages of Octet BLI
No flluidics
– sensor tips (no chips)
– micro-well plates
– easy sample recovery
Label-free
– no fluorescent dyes required
– numerous coupling methods
– covalent and affinity-based
Robust
– Free choice of assay buffers
– Gylcerol and DMSO
– Crude biofluids
Dynamic range
Detectable affinities from pM to mM
Octet BLI Technology
BLI – Technology and FAQs
Overview
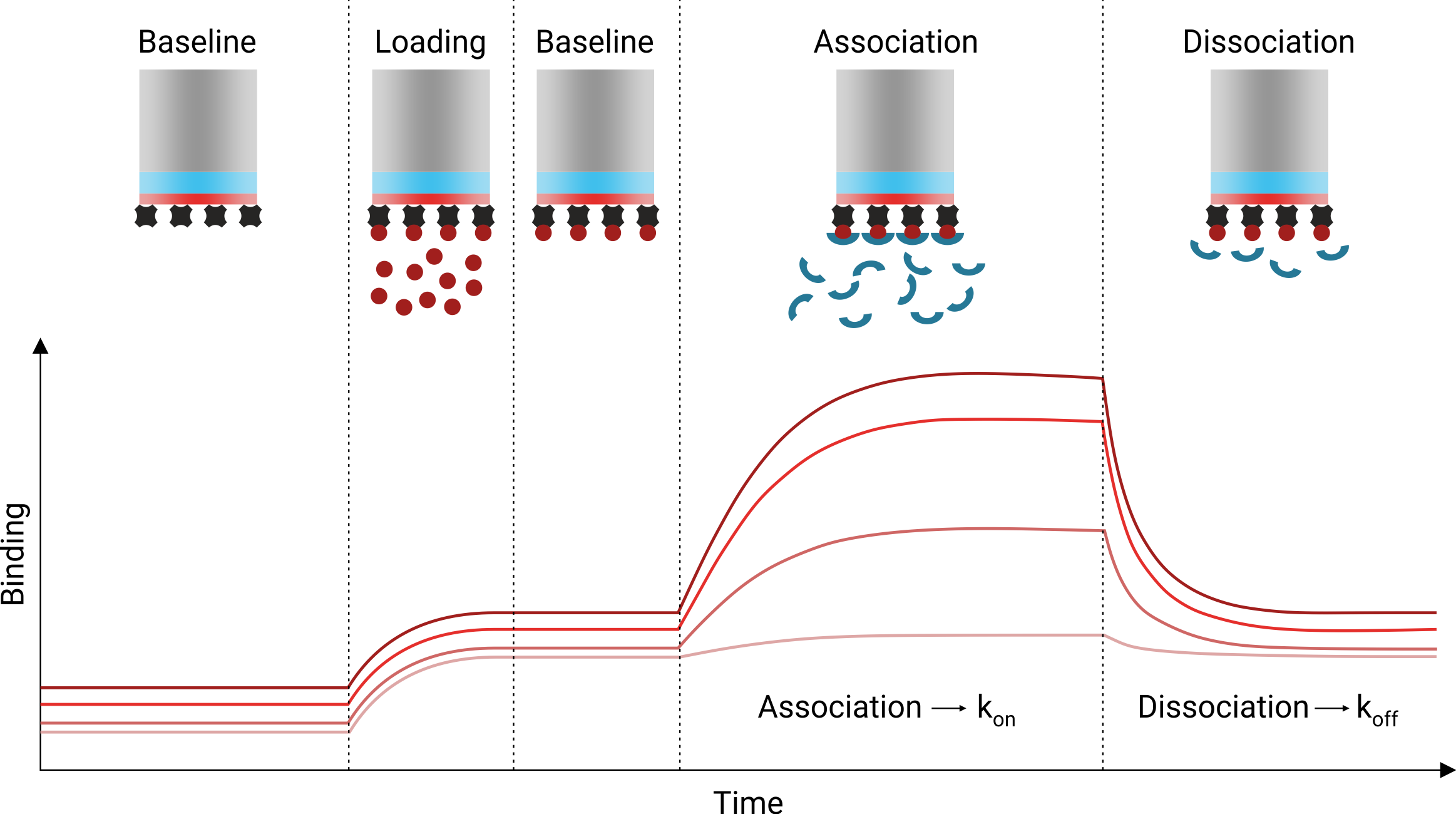
For many molecular interactions it is not only important to know their precise affinity, their thermodynamics, or their stoichiometry. It is often equally important to get detailed knowledge about their underlying kinetics. Two processes constitute the kinetics of a molecular interaction: Association, i.e. the formation of attractive forces between the interacting molecules, and dissociation, i.e. the disengagement of the molecules. In many pharmaceutical and biotech applications, analysis of kinetics is important, for example to tailor the kinetics of therapeutic or diagnostic antibodies specifically towards rapid association or slow dissociation. In order to derive the kinetics of a molecular interaction, the association and dissociation phases of the interaction have to be monitored over time. A very sensitive, robust, and precise way to do measure binding kinetics is Biolayer Interferometry (BLI). This method detects the build-up of molecular complexes on a sensor-tip, which specifically binds one interaction partner. In simple terms, the BLI setup is an inverse SPR (Surface Plasmon Resonance, Biacore ®) setup.
Loading of one interaction partner to the sensor tip can be done, for example, with the well-established biotin-streptavidin system. Initially, the sensor tip is dipped into a blank solution (e.g. buffer) to record a baseline (see figure below). Then, one interaction partner (e.g. a biotinylated antigen) is captured on the sensor tip surface and excess antigen is washed off with buffer. In order to monitor the association, the loaded sensor tip is then dipped into a solution containing the second interaction partner (e.g. an antibody). Finally, in order to monitor the dissociation, the sensor tip is dipped into blank buffer solution again, where the ligand (antibody) will dissociate over time from the coupled target (antigen). Certain sensor tips can also be regenerated and re-used for several experiments. Finally, fitting of the association and dissociation phases then provides the respective kon and koff rates and thus kinetics information.
Importantly, the Octet® BLI system is label-free and does not require the modification of the interaction partners with fluorescent dyes for example. However, immobilization of one interaction partner has to be accepted in turn. The Octet® BLI system is widely used for quantifying and analyzing the kinetics of molecular interactions involving small molecules, peptides, DNA and RNA molecules, proteins, antibodies, up to whole viruses and bacterial cells. In contrast to the Biacore® SPR system, BLI has the advantage that no microfluidics are required, which makes the system more robust and allows for measuring in complex biological liquids like cell lysate, serum, blood plasma, urine, sea water, etc.
Technology
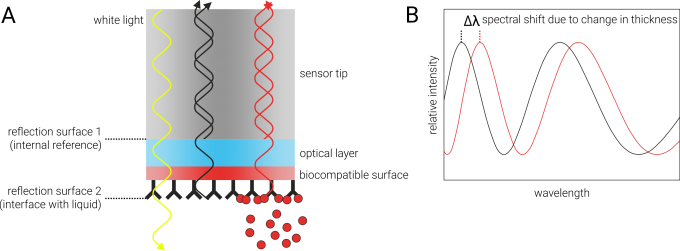
The Octet® K2 BLI platform is based on the optical and label-free analytical technique called Biolayer Interferometry (BLI). BLI analyzes interference patterns of white light that is reflected from two optical layers of a very small (600 µM diameter) tip: One internal reference layer inside the tip and one layer at the interface between the tip and the surrounding liquid (Figure below, panel A). Each reflection generates constructive and destructive interferences that vary with the wavelength (Figure below, panel B, gray curve). Any change at the outer layer of the tip (a biocompatible surface with one interaction partner immobilized on it), for example due to binding of a ligand, leads to different interference patterns at this reflective layer. This, in turn, causes a shift of the interference spectrum to different wavelengths (Figure below, panel B, red curve). From the time-resolved monitoring of this shift, it is possible to derive real-time association and dissociation rates of the ligands in solution to the immobilized interaction partner at the tip surface.
BLI allows for the real-time determination of the interaction dissociation constant (KD), as well as the observed association (kon) and dissociation (koff) rate constants. A wide range of different sensor tip surfaces allows the precisely tailored immobilization of one interaction partner. Common techniques for immobilization are direct surface immobilization using amine-reactive coupling, biotin-streptavidin based coupling, anti-GST– or anti-histidine-tag based coupling, and antibody-based coupling. Thus, BLI using the Octet® system generates a complete kinetic profile of a molecular interaction and by that, for example, help with a more-fine grained analysis of molecular interactions with similar equilibrium affinities.
Here at 2bind, the state-of-the-art the PALL FortéBio Octet® K2 BLI System is used. This BLI system features two parallel measurement channels, a 96-well sample plate format, excellent sensitivity down to analytes of 150 Da, and high reproducibility. A sample consumption of only 200 µL and the possibility to re-use a sample for multiple measurements enables that analysis of precious and difficult to obtain analytes. The Octet® K2 system features a dynamic range of association and dissociation rate constants of six orders of magnitude (kon: 101 – 107 M-1s-1, koff: 10-6 – 10-1 s-1). Affinities (KD) can be determined in the range from 10-3 – 10-11 M. The lower and upper boundaries for quantification are 0.05 µg/mL and 2000 µg/mL, respectively.
Typical applications
Kinetics applications
- Protein-protein interactions
- Antibody characterization
- Antibody-antigen binding
- Protein-small molecule interactions
- DNA-aptamer binding
- Bacteria-antibody interactions
- Virus-like particle-antibody/protein binding
- GPCR-protein binding
Quantitative applications
- Antibody quantitation
- ELISA replacement
- Protein quantitation in crude extracts
- Detection of contaminations
Screening applications
- DNA-aptamer screening
- Small molecule fragment screening (with certain limitations)
- Secondary screening and hit validation (with certain limitations)
- Inhibitor screening
Assay development applications
- Formulation development
- Media development
For examples of such applications, take a look at our Application Database.
Advantages
- Label-free detection method → no fluorescent dyes required
- Free choice of assay buffers → also gylcerol or DMSO possible as solvents
- Complete kinetic characterization → association and dissociation rate constants determinable
- Numerous coupling methods → covalent and affinity-based coupling methods available
- Measure at elevated temperatures → analysis possible up to 40°C
- Measure in crude biological liquids → cell lysate, serum, plasma, environmental samples
- Wide range of binding affinities → range from nM to mM affinities measurable
- Wide range of possible molecules → from large VLPs to, under certain conditions, small molecules
Frequently asked questions
FAQ – General
What is BLI?
What parameters of a molecular interaction can be determined with BLI?
If you are only interested in the steady-state affinity of a molecular interaction, consider using MicroScale Thermophoresis as an alternative. This method allows for the highly precise, robust, and fast determination of the dissociation constant of molecular interactions. If you are interested in the thermodynamic parameters of an interaction, take a look at Isothermal Titration Calorimetry.
What is the slowest dissociation rate that can be measured?
The primary limitation for the measurement of very low dissociation rates is sample evaporation from the microplate wells.
What is the fastest association rate that can be measured?
Why does the vertical axis of BLI plots display a thickness in nm?
FAQ – Samples
How much sample is required for BLI assays?
If these requirements are problematic for your special case, MicroScale Thermophoresis is a great alternative to determine steady-state affinities of molecular interactions with minimal sample consumption.