High-throughput analytics for biologics and small molecules

Do you need dependable particle and protein analysis services? Look no further than our Wyatt DLS Plate Reader 3 platform! The Wyatt DLS Plate Reader 3 is ideal for high-throughput screening applications, thanks to its sophisticated automation features and lightning-fast data gathering. Our experienced scientists utilize the system to provide a variety of diagnostic services for biologics and small molecules, such as isothermal and temperature-dependent particle sizing, solution homogeneity, and aggregation analysis. The platform combines both Dynamic Light Scattering (DLS) and Static Light Scattering (SLS).
Particle sizing via DLS
CUMULANT OR REGULARIZATION ANALYSIS
rH
Hydrodynamic radius shows size of particle in solvated state
PDI
Polydispersity index represents distribution of size populations
SELF-INTERACTION ANALYSES
kD
Diffusion interaction identifies onset of unfolding and impact on colloidal stability
D0 determined from the kD analysis
Theoretical diffusion constant at concentration = 0
SIZE ANALYSIS
Tsize (from growth of rH in cumulants fit)
Temperature at which average particle size begins to increase
Tscattering (from scattering intensity)
Fragments, PROTACs, ions, nanoparticles, peptides, and carbohydrates
Average scattering intensity
Identifies if concentration is too high or low for proper size distribution analysis
Molecular and solution properties
MOLECULAR MASS
MW
Molecular weight of biological particles in solution (e.g. proteins)
SOLUTION VISCOSITY
μ
Resistance of a fluid to flow

What molecular parameters can be obtained with the 2bind DLS/SLS assays?
Hydrodynamic radius
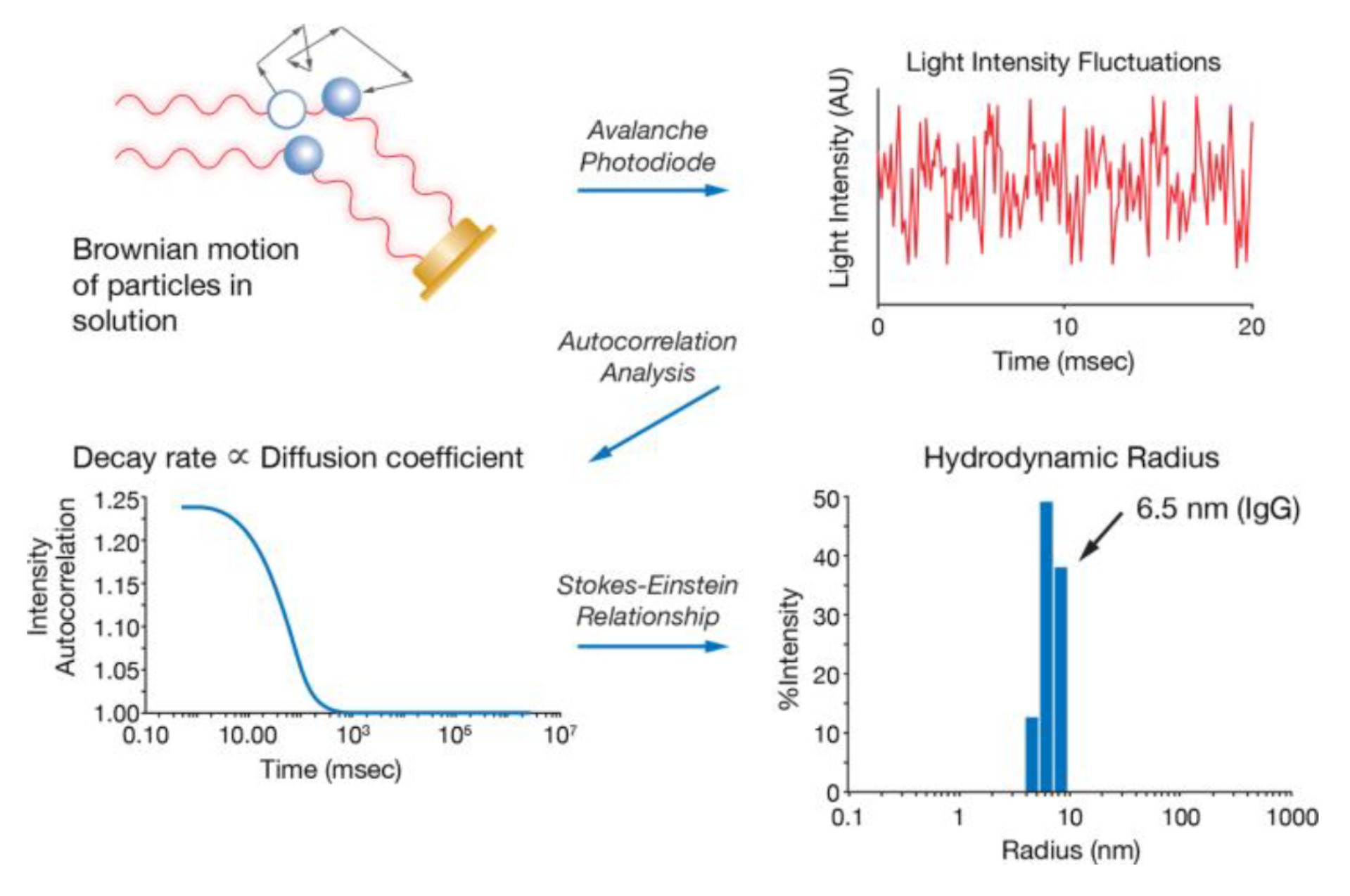
The hydrodynamic radius (rH) is a parameter used to describe the size of particles in solution, taking into account the solvation layer surrounding it. It is related to the diffusion coefficient of the particle and the viscosity of the solvent through the Stokes-Einstein equation, making it a critical parameter for characterizing particles in solutions, particularly for biological and nanoparticle applications.
Dynamic light scattering (DLS) is used to determine the hydrodynamic radius by measuring time-dependent fluctuations in intensity to obtain an auto-correlation function. This function provides information about the size distribution of the particles in solution, from which the rH can be calculated. The analysis can be done using either cumulant or regularization methods, depending on the sample characteristics and desired accuracy.
Polydispersity index (PDI)
Polydispersity index (PDI) is a parameter used to describe the heterogeneity of particles in solution. It is calculated as the ratio of the width of the size distribution to the mean size of the particles. PDI values close to zero indicate a monodisperse sample, while higher values indicate a more heterogeneous sample.
Analysis of the autocorrelation function provides information about the size distribution of particles, from which the PDI can be calculated. PDI values can be calculated using either the cumulant method or the regularization method, depending on the sample characteristics and desired level of accuracy.
Molecular mass
Molar mass is a parameter used to measure the size and weight of particles in solution, particularly large molecules and nanoparticles. It is typically reported in units of g/mol or Da (Daltons).
In addition to DLS, the Wyatt Plate Reader 3 also features static light scattering (SLS) for measuring the molar mass of particles in solution. SLS measures the intensity of scattered light at a fixed angle, providing information about the weight-average molar mass of the particles. Advanced analysis software allows for accurate and reliable measurement of molar mass over a wide range of sample types and concentrations, enabling researchers to gain valuable insights into the properties and behavior of biological and synthetic materials.
Self interaction parameter
The self-interaction parameter (kD) is a measure of the strength of intermolecular interactions in solution, and is important for characterizing the stability and behavior of particles such as proteins and other biomolecules. It is typically reported in units of M-1.
kD is determined from the analysis of second virial coefficient (B22) data using static light scattering (SLS). B22 measurements are obtained by measuring the intensity of scattered light at a range of angles, and can be used to calculate kD based on the concentration and size of the particles in solution. By combining DLS, SLS, and B22 measurements, the Wyatt Plate Reader 3 provides a comprehensive tool for characterizing the stability, size, and intermolecular interactions of particles in solution.
Viscosity
Viscosity is a measure of a fluid’s resistance to flow, and is an important parameter for understanding the behavior of particles in solution. It is typically reported in units of mPa·s or cP.
The Wyatt Plate Reader 3 is capable of measuring solution viscosity using a bead-based method. For this, a known quantity of spherical beads of known size are introduced into the sample solution, and the motion of the beads is tracked using Dynamic Light Scattering (DLS). By analyzing the time-dependent decay of the bead motion, the software can calculate the solution viscosity.
This method is particularly useful for samples that are difficult to load onto a microfluidic chip or are prone to fouling. The Wyatt Plate Reader 3’s bead-based viscosity measurement provides an alternative to microfluidic-based viscosity measurements. The bead-based viscosity measurement method can be used for a wide range of sample types and concentrations, making it a versatile tool for the characterization of biological and synthetic materials.
Tsize
Tsize is a measure of the temperature at which average particle size begins to increase. It is an important parameter for understanding the stability and behavior of particles in solution.
The Wyatt Plate Reader 3 is capable of measuring Tsize using the growth of rH in cumulants fits. Tsize is then determined by observing the temperature at which the average particle size begins to increase. This method is particularly useful for samples that are temperature-sensitive or have a narrow temperature range for optimal stability.
By measuring Tsize, researchers can better understand the stability and behavior of biological and synthetic materials over a range of temperatures, which can inform formulation and processing decisions.
Tscattering
Tscattering is a measure of the temperature at which the intensity of scattered light begins to increase. It is a critical parameter for understanding the stability and behavior of various types of particles in solution.
Tscattering is determined by observing the temperature at which the intensity of scattered light begins to increase. This method is applicable for various types of samples such as Fragments, PROTACs, ions, nanoparticles, peptides, and carbohydrates. This information can help researchers understand the behavior of particles in solution, such as aggregation or precipitation, and optimize formulation conditions to avoid unwanted outcomes.
Wyatt DynaPro III DLS Advantages
Time-efficient
– up to 384-well plates in one run
– high-throughput analysis
– up to 10 000’s of samples
High versatility
– from small molecules to biologics
– artificial particles
– 1 nm to 2 µm particles
Label-free
– Only 25 µl sample
– no labeling
– no immobilization
DLS Technology
Overview
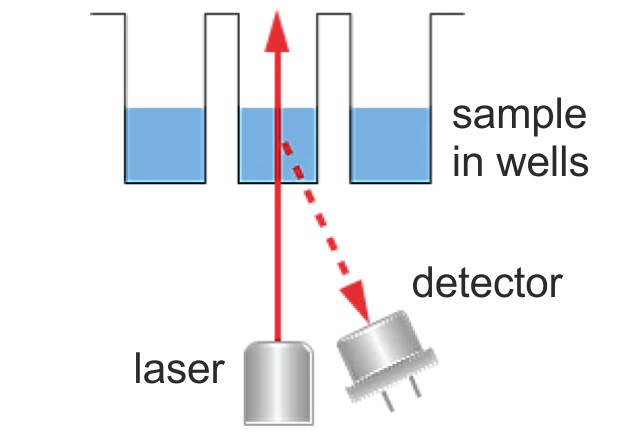 For both static and dynamic light scattering in a well plate, laser illumination and detection take place from below. Brownian motion of particles produces dynamic light scattering signals that look like noise. However, these fluctuations do contain useful information: diffusion coefficients that can be converted to size.
For both static and dynamic light scattering in a well plate, laser illumination and detection take place from below. Brownian motion of particles produces dynamic light scattering signals that look like noise. However, these fluctuations do contain useful information: diffusion coefficients that can be converted to size.Technology
When light hits particles that are smaller than the wavelength of the light, it is scattered in all directions without loss of energy. This effect is known as elastic light scattering. Due to the diffusion of particles in solution (Brownian motion), the scattered light waves interfere with each other in a constructive and destructive manner resulting in intensity fluctuation over time.
Small particles diffuse faster than large ones and the scattered light intensities therefore change faster in a solution with smaller particles than in a solution with larger ones. A digital autocorrelator function now correlates the intensity fluctuation over time, from which diffusion coefficients can be derived. From the diffusion coefficient, the hydrodynamic radius of the sample particles can be determined using the Stokes-Einstein equation.
Brownian motion of particles produces dynamic light scattering signals that look like noise. However, these fluctuations do contain useful information: diffusion coefficients that can be converted to size.
Typical applications
Due to the robust detection method and quick analysis pace, Wyatt PlateReader 3 DLS is very versatile and can be used in a number of contexts:
- Antibody engineering (determine aggregation propensity, get self- and non-specific interactions)
- Pre-formulation and formulation development (determine aggregation propensity, determine thermal stability, perform buffer and excipient screening, characterize particle sizing and solution homogeneity)
- Buffer screening
- Excipient screening
- Process development (determine and compare protein aggregation, characterize particle sizing and size distribution during process scale-up and optimization)
- Biologics development (characterize protein unfolding and aggregation, get self- and non-specific interactions, analyze protein size and homogeneity)
- Small molecule quality control (determine particle sizes after reconstituion, dilution, mixing, isothermally and across temperature gradients)
- Comparability studies (determine particle size distribution across different batches, lots, processes, perform stress testing of biologics)
For more information on these applications or the DLS analysis services by 2bind, take a look at our Application Database or contact our DLS specialists.
Advantages
DLS in general and with the Wyatt Plate reader 3 a in particular offers a great number of benefits:
-
Short measurement time: 384 samples require only 1.5 h for isothermal DLS measurement.
-
Great sample type versatility: All kinds of macromolecular and polymeric sample types can be measured. Sizes down to 1 nm and up to 2 µm can be reliably detected: Peptides, proteins, nucleic acids, lipids and liposomes, artifical polymers, detergents and detergent micelles, viruses and virus-like particles, cell organelles.
-
Deep information content: Get insights into sample quality, abundant species distribution, effects of ligand binding and conformational changes in one experiment.
-
Label free detection: Only 25 µl sample is required, no sample labeling or immobilization neccesary.
-
Wide molecular weight range: Particle sizes from 1 kDa to 1 MDa.
Frequently asked questions
FAQ – General
What are typical applications for the DLS Technology? Is screening possible?
DLS is a very versatile technique since it can be applied to all kinds of biological particles. Proteins are usually the main target for DLS analysis. Another major class of DLS experiments is development of solution characteristics, for example for biologics formulation development or cosmetics.
Due to the fast analysis pace and high throughput, DLS is well suited for screening of buffers, buffer additives or small molecule influence on protein stability and protein unfolding.
How many samples can be measured in parallel?
With our DLS setup, it is possible to measure 384 samples in parallel.
Is it also possible to analyze the stability and unfolding of protein with DLS?
Protein unfolding can be measured with the Wyatt DLS Plate Reader 3 using a technique called temperature ramping. The instrument measures the hydrodynamic radius of the protein at different temperatures, and the resulting data is used to generate a thermal denaturation curve. The unfolding temperature (Tm) can be calculated from this curve, providing insight into the protein’s stability and behavior under different conditions. This information is critical for the development of biopharmaceuticals and other protein-based products.
Is it possible to observe ligand-induced changes of the hydrodynamic radius?
Yes, it is possible to observe the effect of ligand binding on the hydrodynamic radius by monitoring the cumulant radius of the target in the presence of increasing concentrations of the ligand.
Can I characterize protein-protein interactions with DLS?
Yes, it is possible to measure protein-protein interactions with DLS. For this, a continuous variation experiment can be performed, in which the concentrations of the two interaction partners are simultaneously and continuously varied and the mean particle size is determined. Upon binding, the largest particle size will be observed. Note that for this experiment to work, the differences in hydrodynamic radius of the single proteins must not exceed two-fold (or the molecular weight difference must not exceed 5-fold).
FAQ – Samples
How much sample is required?
Wyatt DLS plate reader works with 384 well plates. Thus, only 25 µl of sample volume is required per experiment. And the sample can be easily reused for different experiments after the measurement.
What concentration range of my samples can be measured?
In general, it is possible to analyze protein solutions from 0.1 mg/ml up to more than 20 mg/ml.
What particle size range can be measured?
Particles between 0.5 nm and 2 µm can be measured.
FAQ – Assay Conditions
What is the temperature range for the measurement?
DLS can be measured isothermally at any temperature between 15°C and 95°C. In addition, thermal ramp measurements of DLS and nanoDSF can be done with heating from 15°C to 95°C, with heating rates of 0.1 to 5°C/minute.
Are there any limitations in buffers or additives?
Our sample preparation protocol carefully prevents any dust or particle contamination prior to measurement. In general, DLS is not limited by buffers or additives. However, it must be noted that every particle in the sample will scatter light. Thus, complex solutions might give background scattering signals that prevents successful DLS analysis.