nanoDSF is a biophysical technique used to assess the conformational stability of protein, which essentially means it checks how likely a protein is to remain folded and functional under stressful conditions. It works by monitoring a protein’s intrinsic fluorescence as it responds to thermal or chemical stress. As the protein unfolds under this stress, its fluorescence properties change. The point at which unfolding occurs (“melting temperature”) is indicative of the overall conformational stability of the protein.
nanoDSF can detect even minor changes in a protein's fluorescence, providing a very detailed picture of its conformational stability. It can measure both thermal stability (how a protein behaves with increasing temperature) and chemical stability (how it behaves in the presence of denaturing chemicals). NanoDSF provides a wealth of data, including Tm (the temperature at which half of the protein unfolds), Ton (the temperature at which unfolding starts), the slope of the unfolding curve, and the activation energy of unfolding, all together key thermodynamic parameters for protein stability.
Moreover, nanoDSF can also record even very small changes of protein conformational stability coming from binding of a ligand. This can be used for efficient, high-throughput screening for ligand binding (Thermal Shift Assay, TSA). Importantly, since nanoDSF works with intrinsic protein fluorescence, neither fluorescent labeling, nor the presence of an external fluorescent dye are necessary.
Melting temperature; the temperature at which half of a protein sample is unfolded
Onset temperature; the temperature at which protein unfolding starts
Activation energy of unfolding
Onset temperature of aggregation; the temperature at which protein aggregates start to appear
Difference of melting temperature of a protein in presence and absence of a ligand (e.g. small molecule)
Dose-response format of Tm-diff measurement
2bind’s nanoDSF services are a powerful tool to get to actionable results faster, with higher accuracy, and with higher quality: Selecting the most promising drug candidates early in development (by assessing protein stability early on candidates with the highest stability can be selected, which are more likely to succeed in downstream development stages). Getting an efficient and fast picture of a large ligand collection for possible binding to a target (by in-solution, label-free thermal shift assay).
NanoDSF stands for the nano-format of Differential Scanning Fluorimetry (DSF). It is a fast, robust, high-quality, and – most importantly – label-free and in-solution method for the analysis of protein stability, thermal protein unfolding and melting temperature analysis. Consequently, nanoDSF is a great tool for buffer and formulation screening as well as screening of small molecule compound libraries for influence on protein stability and shifts of thermal melting temperature. Additionally, nanoDSF allows for analyzing the colloidal stability of protein solutions (aggregation).
In general, the intrinsic tryptophan fluorescence of proteins strongly depends on the protein’s 3D-structure and hence the local surroundings of the tryptophan residues. Using chemical denaturants or a thermal gradient, proteins can be unfolded, which leads to changes in their intrinsic tryptophan fluorescence. This translates into fluorescence emission peak shifts and intensity changes. NanoDSF monitors these fluorescence changes with high time-resolution and can reveal even multiple unfolding transitions. NanoDSF is therefore highly successful in antibody development, membrane protein characterization, protein quality control, buffer screening, protein unfolding analysis, and thermal shift assay (TSA) binding screening.
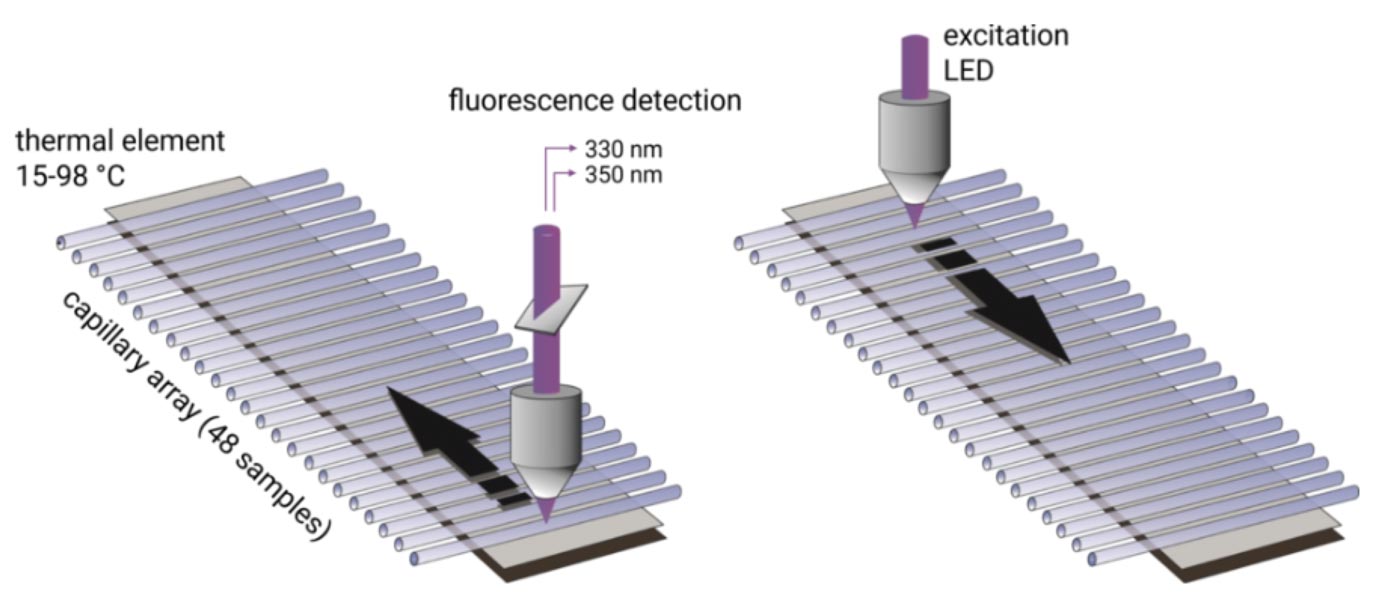
Up to 48 capillaries are filled with only 10 µl of protein sample and are simultaneously scanned at 330/350 nm wavelengths. Melting temperatures are recorded by monitoring changes in the intrinsic tryptophan fluorescence and aggregation onset temperatures are detected via back-reflection light scattering. The samples can be heated to any temperature in the range from 25°C to 95°C. Importantly, samples can be studied without the use of a dye and with free choice of buffer and detergent. Melting temperatures of proteins with a concentration between 5 µg/ml and 250 mg/ml can be analyzed. In order to obtain high quality aggregation onset temperatures, protein solutions with concentrations above 1 mg/ml are required.
The interpretation of nanoDSF data can often be complex, due to different changes of the recorded fluorescence intensity at different wavelengths and shifts of the underlying fluorescence spectra of the denatured protein in relation to the native state. First, the case where the denatured state shows a fluorescence spectrum that is red-shifted in relation to the spectrum of the native state: A red-shift is the most common result of protein denaturation, because the tryptophan residues, which are usually packed inside the hydrophobic core of the protein are now exposed to the hydrophilic solvent environment. This leads to a change of their fluorescence properties towards higher wavelengths. Note that the Prometheus NT.48 nanoDSF device does not measure full fluorescence spectra, but only monitors at the two distinct wavelengths 330 nm and 350 nm. The depicted spectra are only for visualization. In the case of a red-shift upon protein unfolding, the fluorescence signal at 350 nm does not change too much; consequently, the fluorescence intensity progression in the single-wavelength graph is rather linear. However, at 330 nm the fluorescence intensity changes quite drastically due to the red-shift; consequently, the fluorescence intensity decreases with increasing temperature. The division of the 350 nm signal by the 330 nm signal now leads to an inversion of the curve progression, because “no change” at 350 nm is divided by “decrease” at 330 nm, resulting in an increase of the ratio signal. Finally, the mathematical operation of 1st derivate transforms the ratio curve into a classical peak shape. The maximum of the peak corresponds to the melting temperature of the protein (inflection point of the underlying ratio curve).
Second, the case in which the denaturation of the protein leads to a blueshift. This can either be the case for a surface exposed tryptophan, which gets surrounded by hydrophobic residues in denatured “clumps” of protein. Alternatively, a blueshift is sometimes observed for interior tyrosine residues. In the case of a blue-shift upon protein unfolding, the fluorescence signal does not change that much at 330 nm and changes more strongly at 350 nm. Consequently, the fluorescence intensity curves look exactly opposite to the red-shift case: Now, there is not much change in the fluorescence intensity progression of the 350 nm signal, but a pronounced decrease of the 330 nm fluorescence intensity signal. The division of the 350 nm signal by the 330 nm signal results in a similarly oriented ratio curve, because a “decrease” at 350 nm is divided by “no change” at 330 nm. Finally, the mathematical operation of 1st derivate transforms the ratio curve into a classical peak shape. The maximum of the peak corresponds to the melting temperature of the protein (inflection point of the underlying ratio curve). Due to the decrease of the ratio curve, the peak is now inverted and facing downwards.
Third, the case where there is neither a red-shift nor a blue-shift upon protein unfolding (or both effects cancel each other out). In such a case, only the overall fluorescence intensity decreases for both the 330 nm and the 350 nm channels. This translates into decreases in both fluorescence intensity curves in the single wavelength graph. Because there is now a decrease in both wavelengths, the division of both signals leads to a rather flat ratio curve. Consequently, no meaningful 1st derivative curve can be calculated. In such a case, it is best practice to analyze the single wavelength graphs instead of the ratio curve for determination of the melting temperature. Alternatively, the turbidity signal can be analyzed, since it is independent from tryptophan-based fluorescence signal changes.
NanoDSF especially excels in two main areas of research: First, development of therapeutics and biologics. Second: high-throughput thermal shift assay (TSA) ligand screening. Here is a list of typical nanoDSF applications:
NanoDSF offers a number of advantages over traditional fluorimetry approaches. Most importantly, in contrast to standard Differential Scanning Fluorimetry, nanoDSF does not require the use of fluorescent dyes like Sypro Orange: