FPS (Fluorescence Proximity Sensing) enables real-time monitoring of binding events, offering insights into the kinetics and dynamics of molecular interactions. Researchers gain a versatile tool for studying a wide range of interactions, from protein-protein and protein-DNA to small molecule-protein interactions. Target-immobilization relies on DNA-DNA hybridization offering full control of surface densities and enabling the generation of mixed surfaces with two targets immobilized in defined ratios, thereby providing the prerequisites for systematic avidity studies of bivalent monospecific or bispecifc molecules.
FPS is part of the powerful heliX® technology platform, which also offers switchSENSE, kinFRET, and Real-Time Interaction Cytometry (RT-IC) for comprehensive biomolecular interaction analysis. At 2bind, our team of experienced scientists leverages the full potential of heliX® FPS to deliver comprehensive solutions for your research needs. We combine state-of-the-art instrumentation with expert data analysis and interpretation to ensure accurate, reliable, and actionable results
Fluorescence Proximity Sensing (FPS) is a core measurement mode of the versatile heliX® technology platform, enabling researchers to investigate intricate biomolecular interactions from a kinetic perspective. At the heart of FPS lies a fluorescent dye, strategically tethered to a surface-bound molecule of interest. When a target molecule interacts with the immobilized molecule in proximity to the dye it influences the fluorescence intensity emitted by the dye. By tracking these fluorescence fluctuations, FPS provides a direct readout of the kinetics of binding and dissociation, offering insights into the strength, speed, and specificity of the interaction.
FPS is not limited to a specific type of biomolecule. It can be applied to a broad spectrum of interactions, including protein-protein, protein-DNA, protein-small molecule, and even complex multivalent interactions. This versatility makes FPS an invaluable tool for diverse research areas, from drug discovery and development to basic biological research. The heliX® platform's FPS mode is particularly adept at studying binding interactions to surfaces with defined densities or ratios of two targets of interest.
kon (ka)
Association rate constant. Provides information on how fast complexes form; can be used for KD determination.
koff (kd)
Dissociation rate constant. Provides information on how fast complexes dissociate; can be used for KD determination.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
Avidity
Functional affinity, representing the overall strength of an interaction. Influenced by the binding affinity, binding valency, and the structural arrangement of target and ligand.
ΔH
Binding enthalpy. KD values at different temperatures can be used to obtain the binding enthalpy of an interaction via van't-Hoff-plots.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
EC50/IC50
Dose-response data. Ligand concentration that gives half-maximal response or half-maximal activity.
Drug Discovery: Identify and characterize potential drug targets, optimize lead compounds, and evaluate drug efficacy.
Biotherapeutic Development: Analyze the binding kinetics and affinity of therapeutic antibodies and other biomolecules.
Basic Research: Gain deeper insights into fundamental biological processes, such as signaling pathways and protein complex formation.
Diagnostics: Develop novel diagnostic assays with improved sensitivity and specificity.
The helix sensor chips comprise a gold microelectrode to which DNA nanolevers functionalized with the target molecule are tethered. Due to the inherent rigidity of DNA double helices and their negative charge, their orientation on the chip surface can be precisely controlled by applying an electrical potential to the electrode. Depending on the electrical potential, two distinct measurement modes are possible.
First, a static mode:
A constant negative potential repels the nanolevers, maintaining them in an upright orientation. Although being tethered to the surface, the target molecule at the end of the nanolever is presented at a distance from the gold electrode enabling a solution-like behavior. Binding of an analyte to the ligand at the nanolever’s end causes a change in fluorescence intensity due to alterations in the dye’s microenvironment. Frequently, the fluorescence intensity decreases upon binding of the analyte due to collisional quenching and other processes, although anti-quenching effects can be observed as well. Fluorescent dyes are attached to the distal ends of the DNA nanolevers. Two microelectrodes, each equipped with two single photon fluorescence counters, facilitate highly sensitive detection of two distinct colors simultaneously. Up to four different signals can be monitored, enabling real-time referencing and multiplexed measurements.
Second, a dynamic mode:
An alternating potential induces an oscillating movement of the nanolevers. This movement is sensitive to changes in molecular friction caused by differences in size or shape of the nanolevers. The switchSENSE technology represents an alternative to measure real-time kinetics and enables relative measurements of conformation and sizes of analyte-bound and analyte-free targets.

Key questions of assay design typically include the immobilization strategy. heliX® biosensor chips are equipped with single stranded anchor sequences and the most convenient way is the immobilization of target molecules via DNA hybridization. Different anchor sequences attached to electrode 1 and electrode 2 enable targeted immobilization to each spot. Typically, adapter strands carrying the fluorophore and ligand strands are prehybridized. Each electrode is equipped with a two-color detector and interactions of one analyte with up to four target molecules can be measured in parallel.
Immobilization via DNA hybridization is usually the method of choice to immobilize target DNA or RNA sequences. Convenient, gentle and controlled conjugation methods as well as the proFIRE® purifier streamline the production of protein-DNA conjugates with high quality and purity, which can be easily implemented into the heliX® workflow. Ready-to-use kits for coupling to primary amines exist for molecules with usual pIs as well as for low pI molecules. If the target of interest contains a His-tag, coupling can be directed to amines in proximity of the tag. Alternatively, DNA strands can be coupled to thiol groups of free cysteines with high efficiencies and recovery rates.
As an alternative to chemical modifications of the targets of interest, tag-based capturing can be used. Methods and tools for capturing via His tags, biotin, twin-strep-tag, Fc parts and GFP have been developed and are ready to use.

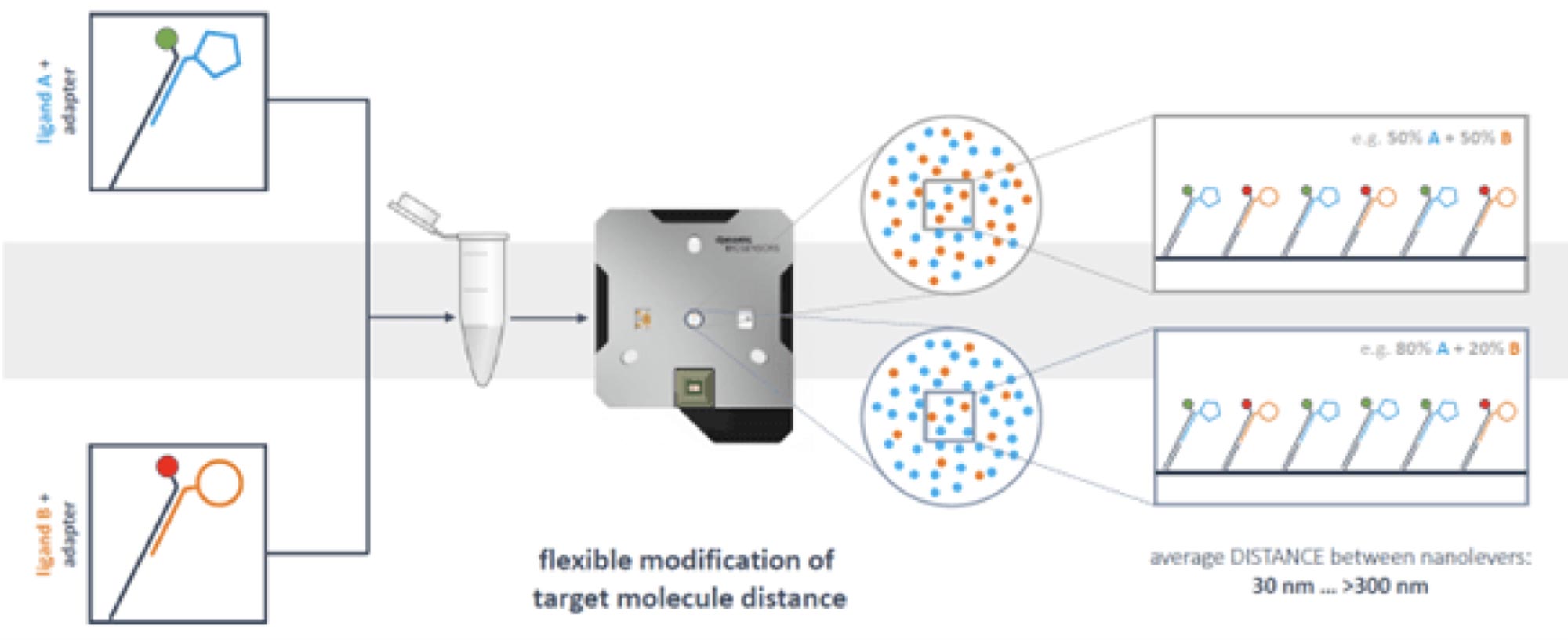
Actually, one chip type allows multiple assay designs. The chips are equipped with unique anchoring sequences in electrode 1 and electrode 2. Dependent on the requirements of a specific experiment, the anchoring sequences can be hybridized with adapter strands of different shape in a functionalization step (see section “What types of adapter strands have been designed up to now?”). The DNA can be conjugated with the ligand, or with a capture molecule. Via the ligand strand sequence, two different target molecules can be applied in the same functionalization step and directed to the two different electrodes. This is useful for measurement of a real-time reference. However, DNA-targeting also enables full control of immobilization densities and spatial distribution of immobilized binding partners on each electrode. Anchoring DNA sequences are fixed on the electrodes in a defined distance. By diluting the fluorescently labeled ligand strand with ligand-free strand hybridized with adapter strand without fluorophore, the density of target on the surface can be reduced and the distance between target molecules can be increased in a highly controlled and reproducible manner. Obviously, it is also possible to load two antigens on the surface. Prehybridization with the alternative fluorophore enables the measurement of interaction of an analyte with two target molecules on the surface simultaneously. The same is true for electrode 2, leading to a total number of four interactions that can be measured in parallel.