Uncover the secrets of your biomolecules with 2bind’s advanced Biolayer Interferometry (BLI) services. BLI, a label-free technology, illuminates the real-time kinetics and affinity of biomolecular interactions. Whether you’re developing antibodies, biologics, or other complex therapeutics, BLI provides crucial insights into binding specificity, affinity, and kinetics, accelerating your research and development.
Our customized BLI solutions utilize the cutting-edge Octet Red384 system, enabling high-throughput analysis for rapid and efficient results. With 2bind’s deep expertise in BLI and life science research, you’ll receive not just raw data, but actionable insights to drive your projects forward. Experience faster turnaround times, exceptional sensitivity, and dedicated support throughout your entire BLI journey.
The Science Behind BLI: Illuminating Biomolecular Interactions. BLI is an optical technique that measures biomolecular interactions in real time. This label-free method eliminates the need for tags or dyes, preserving the natural behavior of your molecules.
How does it work? BLI uses fiber-optical sensor tips, to which the biomolecules of interest are coupled, for example via classical amine-coupling. These sensor tips are then immersed in a solution containing a potential binding partner. When these molecules interact, they bind to the sensor, creating a change in the thickness of the biolayer on the sensor tip. This shift alters the interference pattern of white light reflected from the sensor surface, which is directly measured and translated into real-time kinetic data.
One major difference to classical SPR (Surface Plasmon Resonance) is that the BLI technique does not require any microfluidics or sample flow. Instead, an orbital flow is created by shaking the microwell-plates that contain the samples and into which the optical sensors are dipped. Therefore, BLI works great with complex, viscous bioliquids like serum or cell lysate.
kon (ka)
Association rate constant. Provides information on how fast complexes form; can be used for KD determination.
koff (kd)
Dissociation rate constant. Provides information on how fast complexes dissociate; can be used for KD determination.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
ΔH
Binding enthalpy. KD values at different temperatures can be used to obtain the binding enthalpy of an interaction via van't-Hoff-plots.
c
Molar concentration of biolgics (e.g. proteins, antibodies, antibody fragments) in supernatants, production mixtures, or crude lysates
Epitope specificity
High throughput epitope binning/cross competition assays for determining presence of distinct epitopes and selection of antibodies in drug discovery.
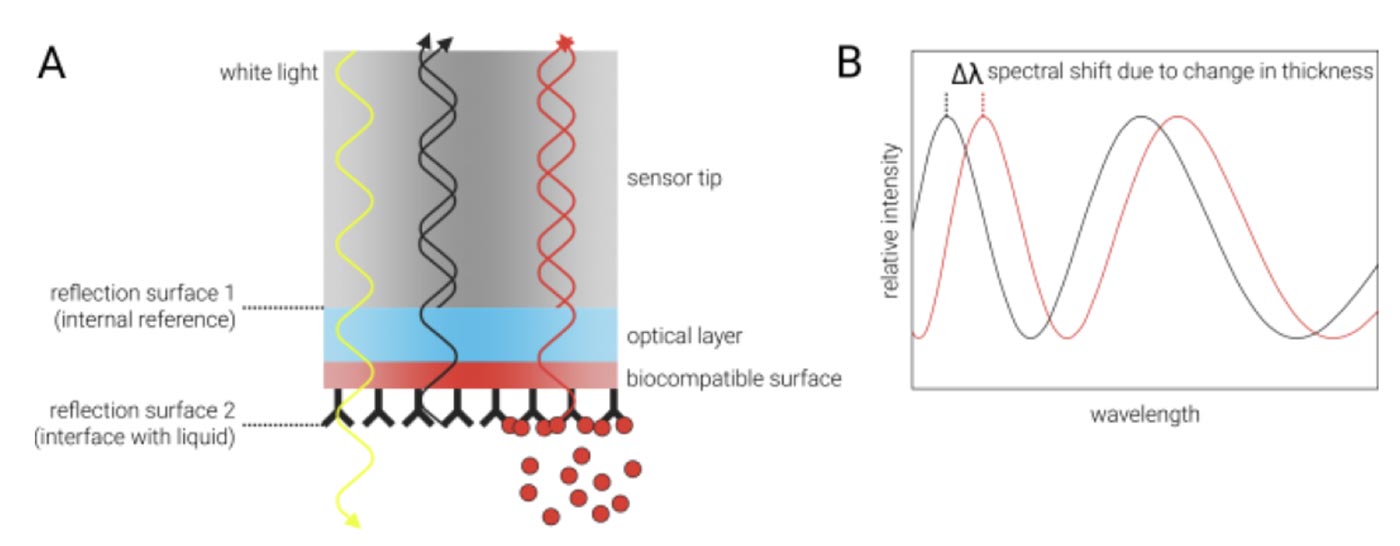
BLI analyzes interference patterns of white light that is reflected from two optical layers on a very small (600 µM diameter) fiber-optical sensor tip: One internal reference layer inside the tip and one layer at the interface between the tip and the surrounding liquid (A). Each reflection generates constructive and destructive interferences that vary with the wavelength (B, gray curve). Any change at the outer layer of the tip (a biocompatible surface with one interaction partner immobilized on it), for example due to binding of an analyte, leads to different interference patterns at this reflective layer. This, in turn, causes a shift of the interference spectrum to different wavelengths (B, red curve). From the time-resolved monitoring of this shift, it is possible to derive real-time association and dissociation rates of the ligands in solution to the immobilized interaction partner at the tip surface.
Loading of one interaction partner to the sensor tip can be done, for example, with the well-established biotin-streptavidin system or via classical amine-coupling. Initially, the sensor tip is dipped into a blank solution (e.g. buffer) to record a baseline (see figure below). Then, one interaction partner (e.g. a biotinylated antigen) is captured on the sensor tip surface and excess antigen is washed off with buffer. To monitor the association phase, the loaded sensor tip is then dipped into a solution containing the second interaction partner (e.g. an antibody). Finally, to monitor the dissociation phase, the sensor tip is dipped into blank buffer solution again, where the analyte (antibody) will dissociate over time from the coupled ligand (antigen). Certain sensor tips can also be regenerated and re-used for several experiments. Finally, fitting of the association and dissociation phases then provides the respective kon and koff rates and thus kinetics information.
