The new Wave in Kinetics

The Creoptix™ WAVEsystem: High-throughput, automated, clog-free, label-free kinetics. Get superior affinity and kinetic data, even for the most challenging samples.
Confidently detect and quantify biological interactions in real-time. Works especially well with small molecule fragments and compounds. Suited for high-throughput screening campaings.
Superior resolution than traditional SPR due to the waveguide technology.
Read more below!
Affinity
LIGAND BINDING
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
ΔH
Binding enthalpy. KD values at different temperatures can be used to obtain the binding enthalpy of an interaction via vant-Hoff-plots.
Kinetics
BINDING KINETICS
ka (kon)
Association rate constant. Provides information on how fast complexes form; can be used for KD determination.
kd (koff)
Dissociation rate constant. Provides information on how fast complexes dissociate; can be used for KD determination.
KD
Equilibrium dissociation constant. Can be obtained by kinetic or classical equilibrium binding analysis. Provides information about the strength but not the dynamics of an interaction.
The WAVEsystem excels in small molecule analysis
For the WAVEsytem, all sizes and affinities are welcome: Screen, rank and characterize fragments, including weak binders with fast dissociation rates and very strong binders, to large particles. Profile kinetics closer to real life. Assess drug performance in undiluted human blood serum and plasma samples. Also, the WAVEsystem cannot just do buffers: Explore solubilization and purification conditions for membrane proteins, including viscous detergents, less common solvents, and different additives. The sophisticated chip technology ensures no clogging, regardless of the sample and solution type.
Screening
The WAVEsystem features 4 parallel lines and rapid kinetics analysis so that screening through large libraries is a breeze!
Ligand binding
Characterize ligand binding for almost any type of sample: Proteins, peptides, challenging membrane proteins, DNA, RNA, aptamers, small molecules, fragments, lipids, particles.
Biologics development
Characterize and quantify your development antibodies at low limits of detection. Not only in buffer, but also in complex biofluids or cell culture media.
Pharmacology
Measure binding kinetics in biofluids like human blood serum or plasma for drug potency assessment. No clogging due to highly sophisticated chip technology.
- Compound screening
- Fragment screening
- Off-rate screening
- Full affinity and single-dose
- Kinetic binding analysis
- Steady-state binding analysis
- Single-dose
- Competition
- Antibody off-rate ranking
- Ligand screening
- Avidity testing
- Ligand binding in biofluids
- E,g, human serum, plasma, liquord
- E.g. cell lysates
- Quantitation in crude extracts
why WAVE and not SPR: the technology
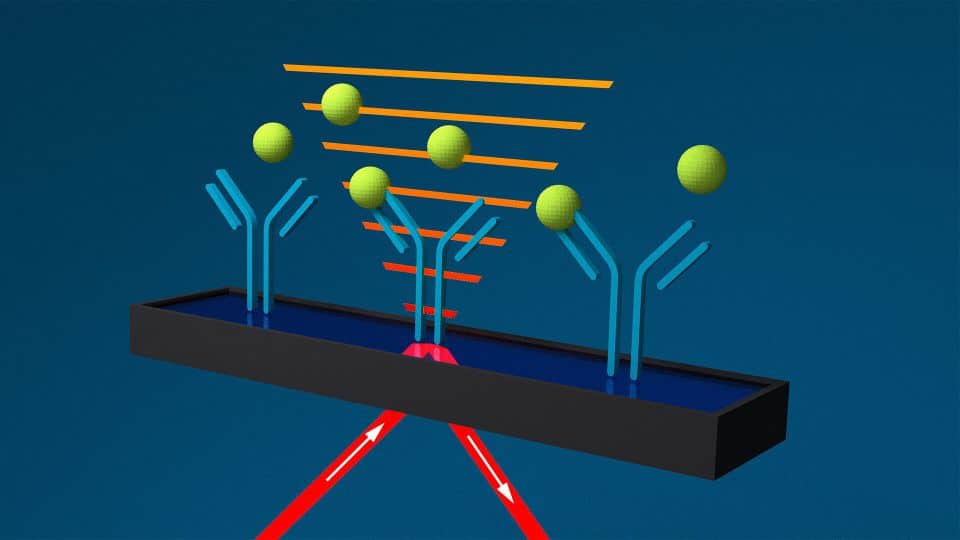
SPR
SPR sensors are usually glass slides coated with a chemically inert metal (black and blue bar). When plane-polarized light hits the metal film at a defined angle (red lines), so-called surface plasmons are excited. Their electromagnetic field (orange bars) is called an evanescent wave and extends beyond the metal surface. Changes in refractive index above the surface, e.g. from ligand-analyte binding, are sensed. This has several limitations:
- Complicated fluidics and detection arrangements necessary
- Detection area limited to evanescent wave field
- Ligand-analyte interaction outside of evancescent field are “invisible”
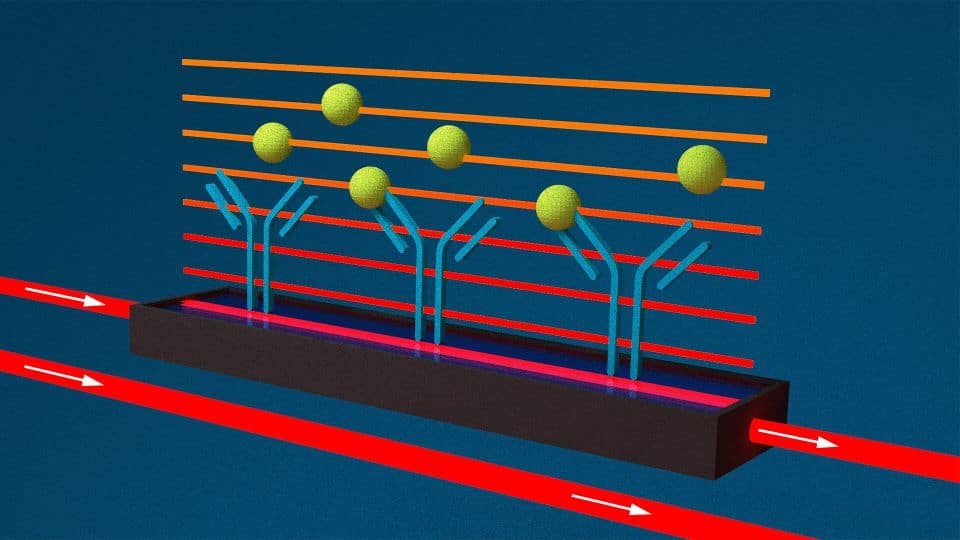
Wave-guide interferometry
Waveguide interferometry also measures changes in the refractive index above a sensor surface. However, light is coupled into a waveguide structure which creates an evanescent field (horizontal bars) across the full length of the sensor surface. Therefore, all binding events across the entire sensor surface contribute to the total measurement signal. This setup has clear advantages over traditional SPR:
- Significantly enhanced detection sensitivity
- Detection across the whole surface
- More shallow field penetration in the solution
- Less artifacts by bulk refractive index changes
- Better signal/noise ratio
Evanescent field in SPR
Waveguide setup
GCI: Grating-coupled interferometry
Classical waveguide interferometer setups (see above) have one problem: The accurate alignment of sample and reference beam is extremely difficult. Grating-Coupled Interferometry (GCI) now solves this issue and builds the core of Creoptix´s WAVEsystem.
In GCI, a laser beam required for creating the evanescent field above the sensor surface is coupled into the waveguide below the sensor via a grating. Now, the reference beam is similarly coupled into the waveguide, leading to interferences between sample and reference beams within the waveguide. Once an analyte binds to the immobilized ligand, the refractive index change translates to a phase shift in the sample beam. This generates a high-resolution, time-dependent and robust phase shift in the interference pattern. This interference shift is then converted to the classical kinetic sensorgrams as in SPR.
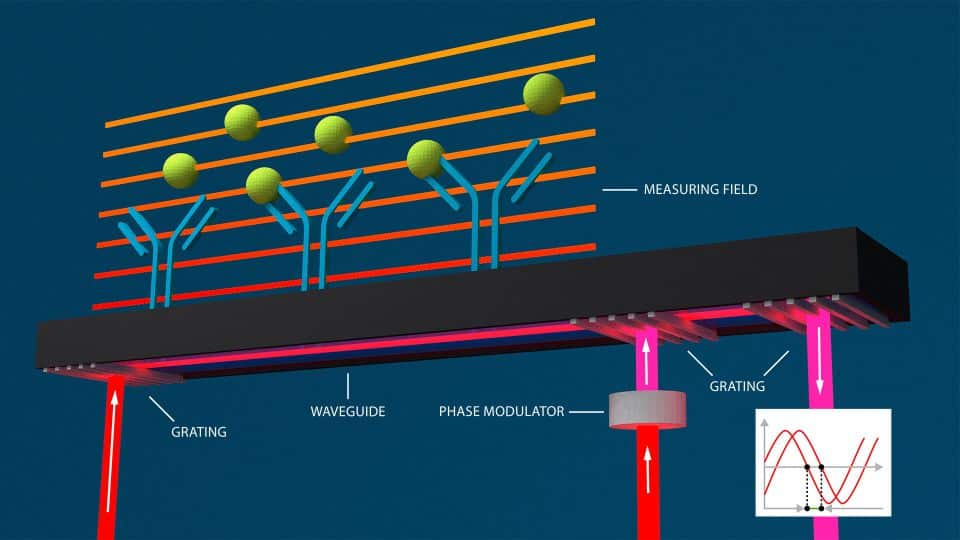
Detection setup of GCI
WAVEsystem Advantages
Broadest kinetic range
Quantify binding affinities (KD) from low pM to high µM with confident kinetic analysis.
Tight binders welcome
All sizes welcome
Kinetic profiling closer to real life
Assess drug performance in undiluted human blood serum and plasma samples.
More than just buffers
Explore more solubilization and purification conditions for membrane proteins, including viscous detergents, less common solvents, and different additives.
No clogging, regardless of size
Analyze and characterize larger molecules (VLPs, liposomes) and aggregates (fibrils) without clogging-risk.
Frequently asked questions
FAQ – General
What is the difference between SPR and GCI?
Both technologies are based on evanescent field sensing. The major difference between GCI and SPR is that in GCI the light travels in a waveguide underneath the sensor surface, pretty much like in an optic fibre. Because of this, the evanescent wave in GCI travels along the entire surface of the chip and is not static like the SPR, in which only around 100 nm along the chip are “sensed”. This means that in GCI the evanescent waves “senses” a lot more interactions than SPR plasmons do. Thus, GCI is inherently more sensitive than SPR.
What surface chemistries can be used for ligand immobilization?
All common immobilization strategies used also in SPR can be directly transferred to Creoptix™WAVE sensors chips: Amine-coupling, Ni-NTA-coupling, Streptavidin-Biotin capture, and others.
What are k-on and k-off ranges that can be measured with the Creoptix™ WAVEsystem?
Association rates constants range from 103 to 5·107 M-1s-1 for small molecules and 103 to 3·109 M-1s-1 for large molecules. Dissociation rate constants can be measured in the range between 10-5 and 10 per second.
What is the molecular weight (MW) limit for analytes?
Analyte:ligand molecular weight ratios up to 1:1000 are possible.
Is the WAVE system compatible with crude sample material?
Absolutely. By combining the microfluidics and the biosensor in a single disposable cartridge, the Creoptix™ WAVE system offers crude-sample robustness normally only achieved with plate-based assays. Compatible biofluids include 100% serum or plasma, cell lysates, crude membrane preparations, large drug targets, virus-like particles (VLPs), liposomes, and aggregates (fibrils); all without the risk of clogging the system.

